Trabeculectomy is the most commonly performed surgical procedure for glaucoma in the United Kingdom and worldwide. Modifications to the technique have been made since its introduction in 1963, perhaps the most significant being the adjunctive use of mitomycin-C (MMC), which has increased the longer-term intraocular pressure-lowering effect of the operation [1,2].
Use of MMC as an adjunct to trabeculectomy was initially described in 1983 by Chen [3], and has been adopted by the majority of surgeons since the late 1990s. However, the use of MMC can lead to significant and sometimes sight-threatening complications; poor surgical technique and higher concentrations of MMC increase this risk.
We present a case of a near miss where an incorrect concentration of MMC was prepared for intraoperative trabeculectomy use due to a pharmacy error.
Case report
A 48-year-old man attended the operating theatre at North Devon District Hospital (NDDH) to undergo a left eye trabeculectomy with 0.2mg/ml MMC under general anaesthesia. During anaesthetic induction, as per normal local practice, the syringe containing the MMC solution, which had been prepared that day by the hospital pharmacy, was checked by the surgical team. The hue of the solution was noted to be an unusually deep purple / blue. Via an immediate telephone conversation, the surgeon received an assurance by the lead pharmacist that the correct procedural process had been followed in preparing the MMC solution. Unconvinced, the surgeon asked him to attend the operating room in person to examine the MMC syringe. The pharmacist agreed the colour was of an unusually deep hue, but did not have concerns regarding its use. Still unconvinced, the surgeon decided not to proceed and the patient was recovered without having undergone the operation.
Subsequent investigation proved the actual concentration of the MMC solution to be 1.0mg/ml instead of the requested 0.2mg/ml.
The incident was reported immediately as a near miss on the local incident reporting system (DATIX) for further investigation and a yellow card Medicines and Healthcare products Regulatory Authority (MHRA) form was completed, which is devised for identifying and collecting information on adverse drug reactions, defective medicines and incidents involving medical devices etc.
Preparation and checking procedure of MMC solution
MMC is manufactured in powder form for hospital use and delivered in glass vials containing 2mg, 10mg or 40mg MMC. The powder has a deep blue / purple colour. Locally, the 2mg vial is reserved for use during trabeculectomy surgery.
- Prior to the surgery date, the pharmacy receives a hand-written prescription for 0.2mg/ml MMC from the ophthalmologist.
- The prescription is entered and recorded on a computer database by a pharmacist, which generates a worksheet for the preparation task.
- A second pharmacist selects a 2mg vial from the cytotoxic drug storage cupboard, checks the expiry date and records in a hand-written form on the worksheet the vial dose and batch / serial number. A tray is prepared containing the MMC vial, 10ml of sterile water, the original hand-written prescription and the computer-generated worksheet.
- A third pharmacist makes an additional check that the appropriate vial dose has been selected and that it is within its expiry date. The tray is transferred to a controlled sterile preparation room into which entry is limited to two personnel.
- Inside the controlled room, two pharmacy technicians make a further check of the vial and paperwork; yet another check is made via CCTV and an audio system with a colleague outside the room. They then prepare the 0.2mg/ml MMC solution by mixing the 2mg MMC vial with 10ml sterile water. 1ml of this final dilution is drawn up in a 5ml syringe which is capped by the user and to which is attached a sticky patient identity label and the MMC dose.
- The syringe is sent outside of the controlled room where it is inspected again by a pharmacist, who checks the solution colour and clarity.
- Finally, the prepared syringe solution is transferred into an opaque pink plastic bag (to prevent denaturation of the MMC by prolonged exposure to ultraviolet light).
Pharmacy error
An investigation concluded that the error stemmed from a 10mg vial being selected instead of a 2mg vial. Reconstitution with 10ml sterile water created a solution of 1.0 mg/ml, which was five times the strength requested and would have accounted for the unusually deep blue / purple hue (Figures 1 and 2).
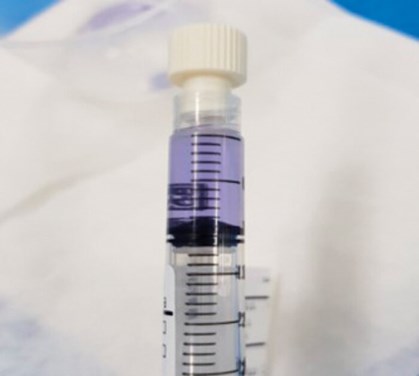
Figure 1: Deep purple hue of MMC.

Figure 2: Normal hue of MMC.
Three main contributing factors were identified:
- The 10mg and 2mg vials are usually segregated in the stocking area. It appeared that the stock had been mixed up.
- The multiple human checks failed to identify that the incorrect vial dose had been selected.
- The IT system was not equipped to auto-select the vial dose in accordance with its intended use, i.e. to instruct the technician to choose a 2mg vial for trabeculectomy.
Discussion
MMC has been widely adopted for its anti-scarring properties in order to improve the efficacy of trabeculectomy and glaucoma tube surgery, minimise re-growth after pterygium excision and maintain a patent fistula following nasolacrimal surgery [4,5,6].
In ophthalmic surgery it is used in concentrations ranging from 0.1mg/ml to 0.5mg/ml [6]. It is also used to treat non-ophthalmic cancers, e.g. bladder cancer, where concentrations are significantly higher, up to 40mg [7,8].
The consequences of an MMC concentration error are potentially very serious. To the best of our knowledge we are not aware of a higher concentration of MMC than 0.5mg/ml used during trabeculectomy or any complications reported in the literature due to it but a very high concentration of MMC, like 1mg/ml in our case, would likely result in serious ocular complications.
There are multiple reports of scleromalacia, scleral necrosis, conjunctival ischaemia leading to bleb leaks, blebitis and endophthalmitis and hypotony following trabeculectomy augmented with MMC concentrations ranging from 0.2-0.4mg/ml [9,10,11].
The requirement to prepare different concentrations of MMC solution according to its indication is a challenge to a pharmacy service charged with serving multiple specialties and it mandates robust preparation and verification procedures. This case exposed a weakness in the verification process of MMC preparation, which has since been corrected. The hospital IT system has also been updated to add a further layer of safety.
References
1. Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol 1968;66:673-9.
2. Yoon PS, Singh K. Update on antifibrotic use in glaucoma surgery, including use in trabeculectomy and glaucoma drainage implants and combined cataract and glaucoma surgery. Curr Opin Ophthalmol 2004;15:141-6.
3. Chen CW. Enhanced intraocular pressure controlling effectiveness of trabeculectomy by local application of mitomycin C. Trans Asia-Pacific Acad Ophthalmol 1983;9:172-7.
4. Nair AG, Ali MJ. Mitomycin-C in dacryocystorhinostomy: From experimentation to implementation and the road ahead: A review. Indian J Ophthalmol 2015;63(4):335-9.
5. Martins TG, Costa AL, Alves MR, et al. Mitomycin C in pterygium treatment. Int J Ophthalmol 2016;9(3):465-8.
6. Foo VHX, Perera SA, Htoon HM, Welsbie DS. Aqueous shunts with mitomycin C versus aqueous shunts alone for glaucoma. Cochrane Database of Systematic Reviews 2015;9.
7. Shelley MD, Wilt TJ, Court J, et al. Intravesical bacillus Calmette-Guerin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: a meta-analysis of randomized trials. BJU Int 2004;93:485-90.
8. Jeong CW, Jeon HG, Kwak C, et al. Comparison of 30 mg and 40mg of mitomycin C intravesical instillation in Korean superficial bladder cancer patients: prospective, randomized study. Cancer Res Treat 2005;37(1):44-7.
9. Akova YA, Koç F, Yalvaç I, Duman S. Scleromalacia following trabeculectomy with intraoperative mitomycin C. Eur J Ophthalmol 1999;9(1):63-5.
10. Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol 1993;116(3):314-26.
11. Singh J, O’Brien C, Chawla HB. Success rate and complications of intraoperative 0.2 mg/ml mitomycin C in trabeculectomy surgery. Eye (Lond)1995;9(Pt 4):460-6.
COMMENTS ARE WELCOME





