Corneal ulceration caused by Acanthamoeba is on the rise, and recent publications indicate an outbreak in the UK over the last few years [1]. Since Acanthamoeba keratitis often presents with atypical features, diagnosis from slit-lamp examination alone can often be inconclusive [2]. Here are some useful tips that can help in making the diagnosis and starting treatment.
1. Clues from the clinical history
Ascertain whether the patient may have any of the following key risk factors that are associated with Acanthamoeba keratitis [1]:
- Swimming, showering, bathing or hot tub use whilst wearing contact lenses (CLs).
- Poor contact lens hygiene such as not washing hands before handling CLs, or washing CLs in tap water.
- Use of high water content, ionic hydrogel CLs (i.e. FDA Group 4 CLs).
- Use of oxipol CL disinfection solution.
2. Clues from the clinical examination
Presence of the following features within the cornea at slit-lamp examination may also indicate infection with Acanthamoeba [3]:
- Dendritic-type shape of ulcer within the corneal epithelium in a CL-wearer.
- Peri-neural infiltrate or peri-neuritis.
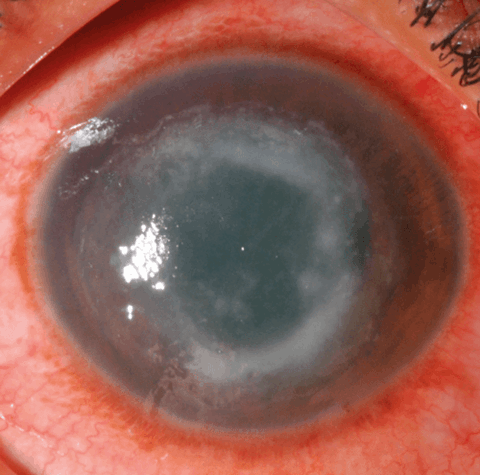
- Ring shaped infiltrate (Figure 1).
Figure 1: Ring infiltrate in the stroma of a patient with culture-positive Acanthamoeba keratitis.
3. Imaging techniques: in vivo confocal microscopy (IVCM)
IVCM imaging can aid in rapidly identifying Acanthamoeba in situ in the patient’s cornea [4] – do the imaging before corneal scraping to increase the chance of seeing the parasite within the cornea. Acanthamoeba cysts usually appear in IVCM images in three ways: double-walled cysts (Figure 2), bright spot sign (Figure 3), or signet-ring appearance [5]. Occasionally, cysts can form lines or clusters and can accumulate in Bowman’s membrane [5,6]. Very rarely, early on in the course of infection Acanthamoeba can also appear as larger trophozoites in IVCM images, however trophozoites can have a highly variable appearance, occasionally with visible pseudopodia [7,8].
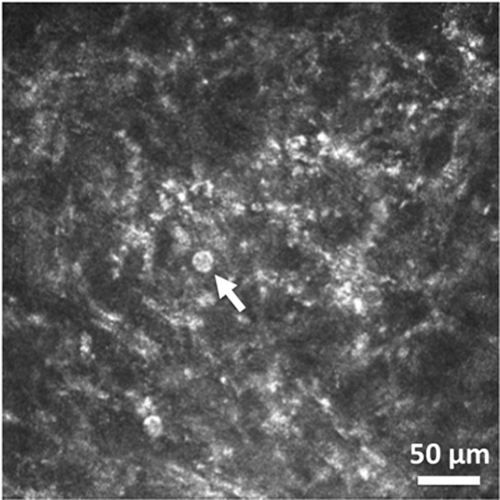
Figure 2: In vivo confocal microscopy image of double-walled cyst (shown by arrow) in corneal stroma in
culture-positive Acanthamoeba keratitis (image obtained using HRT3 laser scanning in vivo confocal
microscope with Rostock Corneal Module, Heidelberg Engineering, Heidelberg, Germany).
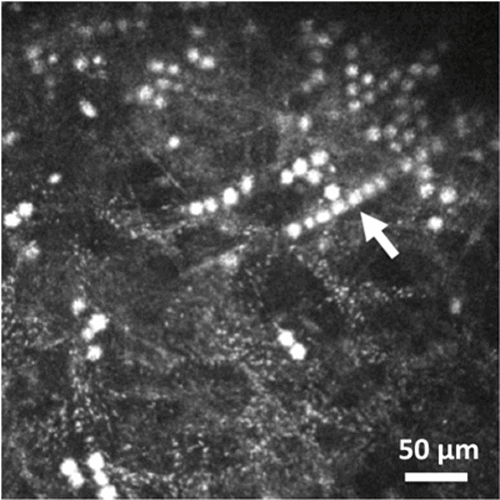
Figure 3: HRT3 in vivo confocal microscopy image showing cysts appearing as
‘bright spots’, with some cysts forming a line (shown by arrow).
4. Corneal scraping for microbiological diagnosis [9]
Scrape the corneal epithelium at the active site of ulceration using a sterile instrument (e.g. sterile green needle 21G, or no. 15 bard parker scalpel blade). Use the side of the needle bevel, or gently use the scalpel blade to remove cells from the base and leading margin of the ulcer. Then place the sample on to the surface of non-nutrient agar (to be seeded with E. Coli in the microbiology lab). After the sample has been placed on the agar plate, discard the needle and use a new needle for taking any further sample from the corneal surface. In addition, place some corneal material on to blood agar and sabouraud agar plates to diagnose bacterial or fungal infection respectively. Also, if enough material is present, apply some to the centre of a sterile glass slide and send to the microbiology lab to identify any organism present (Figure 4), e.g. using gram stain. Finally, consider sending corneal swabs from the surface of the ulcer for polymerase chain reaction (PCR) testing for Acanthamoeba and herpes simplex virus (HSV) if your microbiology lab offers this service.

Figure 4: Appearance of double-walled cysts within corneal scraping placed on glass slide
and viewed immediately after scraping was performed with light microscopy.
5. Treatment options
Commence intensive one hourly eyedrop therapy, initially with polyhexamethylene biguanide (PHMB) 0.02% (or chlorhexidine 0.02%) and propamidine 0.1% (Brolene, May and Baker, Dagenham, UK) day and night for 48 hours, reduced to one hourly daytime only for the following 72 hours, then two hourly for three to four weeks [3]. Dual therapy is useful since many strains of Acanthamoeba may be resistant to one agent alone. Voriconazole 1% eyedrops may also have an anti-acanthamoebal effect, but may be less effective than the biguanides or Brolene [3]. Consider analgesics such as oral nonsteroidal anti-inflammatory medication if the patient has pain associated with the ulcer. Arrange follow-up to review the patient again within a few days to ensure evidence of improvement / response to treatment from symptoms and clinical examination, then drop frequency may be reduced to two hourly daytime only for three to four weeks (as per individual patient response) [3]. Once anti-acanthamoebal therapy has been commenced, if there is prolonged persistence of inflammation, there may be a role for topical steroids, however, this should be performed under the supervision of the cornea consultant since suppression of the immune response in the cornea may sometimes worsen the situation [3]. Monitor microbiology culture / gram stain results since in a small number of cases, mixed infection may be present with another organism, e.g. bacteria, fungi or HSV, that may require additional antimicrobial / antiviral treatment [3]. In case of severe infection, refer to the cornea specialist, since corneal transplantation may be required.
References
1. Carnt N, Hoffman JJ, Verma S, et al. Acanthamoeba keratitis: confirmation of the UK outbreak and a prospective case-control study identifying contributing risk factors. Br J Ophthalmol 2018;102:1621-8.
2. Tu EY, Joslin CE. Recent outbreaks of atypical contact lens-related keratitis: what have we learned? Am J Ophthalmol 2010;150:602-8.
3. Dart JK, Saw VP, Kilvington S. Acanthamoeba keratitis: diagnosis and treatment update 2009. Am J Ophthalmol 2009;148:487-99.
4. Chidambaram JD, Prajna NV, Larke NL, et al. Prospective study of the diagnostic accuracy of the in vivo laser scanning confocal microscope for severe microbial keratitis. Ophthalmology 2016;123(11):2285-93.
5. Chidambaram JD, Prajna NV, Palepu S, et al. In vivo confocal microscopy cellular features of host and organism in bacterial, fungal and Acanthamoeba keratitis. Am J Ophthalmol 2018;190:24-33.
6. Yokogawa H, Kobayashi A, Yamazaki N, et al. Bowman’s layer encystment in cases of persistent Acanthamoeba keratitis. Clin Ophthalmol 2012;6:1245-51.
7. Shiraishi A, Uno T, Oka N, et al. In vivo and in vitro laser confocal microscopy to diagnose acanthamoeba keratitis. Cornea 2010;29:861-5.
8. Labbé A, Khammari C, Dupas B, et al. Contribution of in vivo confocal microscopy to the diagnosis and management of infectious keratitis. Ocul Surf 2009;7:41-52.
9. Leck A. Taking a corneal scrape and making a diagnosis. Community Eye Health Journal 2015;28:8-9.
Acknowledgments:
Images shown are courtesy of Wellcome Trust research study conducted by the author at Aravind Eye Hospital, Madurai, India & London School of Hygiene and Tropical Medicine, UK (WT grant no. 097437/Z/11/Z).
COMMENTS ARE WELCOME