Let’s face it, patients with conjunctivitis don’t always produce the most stimulating consultations and most of the time we can manage them in auto-pilot. The prospect of delving into such a patient’s sexual history is not overly appealing, but this article will explore why you should, at least occasionally! I’ll outline an approach to adult Chlamydial and Gonococcal conjunctivitis and some tips for the next time you find yourself in a sticky situation.
Why is it important?
Although uncommon, sexually-transmitted conjunctivitis is increasing in incidence [1]. Not only are these conditions sight-threatening, but they serve as indicators for systemic disease, with as many as 77% noted to have co-existent genital infection [2]. This degree of co-infection suggests conjunctivitis largely arises from autoinoculation of genital secretions, though ‘direct’ infection is also described [3].
These infections also disproportionally affect the young and sexually active (with a male skew), who are frequently ill-informed with regards to their sexual health [1,4]. This provides a golden opportunity for effective, targeted health promotion.
When should I be suspicious?
One answer is, in anyone that is sexually active, and to know that you need to be taking sexual histories! Some of us became ophthalmologists to escape these topics, but appropriate questioning is very informative, especially given the high proportion of people with co-existing genital symptoms. There are myriad potential questions, but the following are probably most relevant:
- Are they sexually active?
- Do they have one or multiple partners?
- What, if any, contraception is used?
- Are genital symptoms present? – e.g. dysuria, discharge, discomfort.
It is good practice to consider who accompanies the patient when broaching these topics, and how you approach the transition. Warning shots are useful, as lurching in with intimate questions can be jarring.
Suspicions should be raised too when you are confronted with classical symptomology or exam findings that don’t quite fit with bacterial, viral or allergic syndromes.
Gonococcus typically produces an acute, unilateral red eye with profuse mucopurulent discharge (recurring within minutes). Patients may also describe pain, blurred vision, photophobia and malaise. Significant periocular swelling is not uncommon; often to the degree that pre-septal cellulitis is a valid differential [1]. Conjunctival reaction is papillary or follicular, and you may see chemosis, sub-conjunctival haemorrhage and purulent discharge in the fornices.
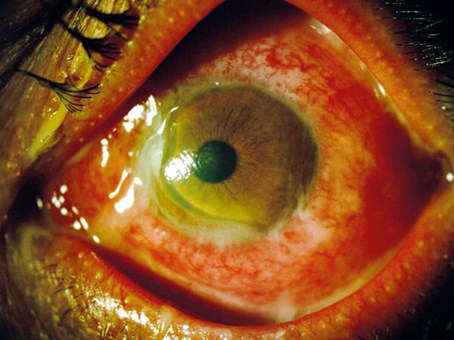
Figure 1: Gonococcal conjunctivitis in the left eye with acute peripheral corneal thinning nasal and inferiorly. Also note hyperaemia, chemosis and purulent discharge in the fornix and matting the eyelashes. Reproduced with permission [11].
Anterior chamber activity is rarer, as are significant corneal changes. These can be devastating, however, with fulminant keratitis, pseudopterygia (conjunctival adhesion to the cornea) and corneal thinning and melting [5] (Figure 1). Orbital features can occur, such as ophthalmoplegia and proptosis, and imaging may be required to exclude focal collections [6].
Chlamydial conjunctivitis is more often bilateral (a third [2]), and produces watery or mucoid discharge. Symptoms may be persistent and have already responded poorly to topical antibiotics. Haemorrhagic discharge is specific in neonates, and potentially in adults [7]. The conjunctivitis tends to be follicular, with chemosis and pseudomembranes, and may involve the bulbar conjunctiva as well as tarsal. Mild corneal inflammation is expected, but clinically important corneal or conjunctival scarring is much rarer [8]. Importantly, many of these features mirror adenovirus, so vigilance and a clear history are vital.
How do I manage them?
Identifying the organism is crucial. On occasion, it may be appropriate to treat complicated gonococcal disease empirically, but confirmation is desirable and usually achievable. Results generally are not immediate so a pathway for recalling or contacting patients is required, and it is important to be frank about your suspicions.
For chlamydia identification, polymerase chain reaction (PCR) is the most helpful test. Infected cells are needed for this, and are acquired through a reasonably rough fornix swab, with a specific kit. These tests are widely available through local microbiology departments and results typically take 24-48 hours. Culture by contrast, is difficult, takes longer, and is rarely performed.
There are more options when testing for gonococcus. Ideally, PCR and culture are performed from a forniceal pus swab, in a similar fashion to chlamydia. PCR takes roughly 24-48 hours, and culture up to five days. Different centres will have their own protocols. Culture has the benefit of providing sensitivities, which is important from a public health perspective and for treatment resistant cases, but the lengthy turnaround is limiting. If rapid confirmation is required, Gram staining of a pus sample spread onto a slide may be performed. This lacks sensitivity but may identify the organism within a few hours, which can be invaluable in severe cases with diagnostic uncertainty.
Systemic therapy is needed to eradicate these organisms, and excellent treatment guidelines are provided by The British Association for Sexual Health and HIV (BASHH). Oral Doxycycline 100mg BD for seven days is first-line for Chlamydia, and only lubricants are recommended topically [9]. Single dose Azithromycin has been replaced due to a concerning rise in macrolide resistance among co-morbid mycoplasma genitalium infections.
Intramuscular Ceftriaxone 1g is preferred for Gonorrhoea [10], and the role of topical therapy is debated. Some feel the quantity of discharge renders such measures ineffective, but others would suggest them supplementally even in simple cases. Corneal or anterior chamber involvement certainly necessitates intensive regimens, however [8]. Lavage as a practice is un-evidenced, but infrequent bathing for comfort is sensible.
For cases complicated by corneal changes, uveitis or severe soft tissue swelling, admission may be warranted and it is prudent to liaise with your infectious diseases (ID) colleagues. Treatment can be initiated on gram stain confirmation and intensified with regular IV cephalosporins and hourly topical agents.
And non-ophthalmic management? (blasphemy?!)
Optimal management requires inter-specialty working, and non-ophthalmic care is best directed by genito-urinary medicine (GUM), ID or sexual health, depending on local pathways (certainly worth reading!)
Regardless of whom by, and potentially during an admission, the patient should be offered:
- Other site testing (genitalia, pharynx, rectum) and potentially bloods (BBVs, syphilis)
- Contact tracing and partner notification
- Test of cure (gonorrhoea especially)
- Advice to abstain from all sexual activity until completion of doxycycline or one week after once only treatments
- Advice regarding contraception and safe sex practices.
Admittedly, this is a big ask and fulfilling all these is certainly not expected of us. As a minimum though, we should be mentioning the above steps, encouraging attendance to the referred specialty, emphasising abstention and providing further information. Helpfully, BASHH produce superb leaflets which can easily be stocked in-house. These are freely accessible at: www.bashhguidelines.org/patient-information-leaflets/
I hope this article has stimulated some thought and provided a few pointers for an often-overlooked set of conditions. Take home points include:
- Don’t be BASHHful with sexual histories
- Maintain a low threshold of suspicion for the typical picture and demographic
- Be vigilant for complications and escalate the case to seniors and other specialties if you are concerned
- Input from sexual health services is always desirable, and there are simple things you can do to better serve your patients (like leaflets!).
Lastly, Table 1 compares and contrasts some of the salient features of both conditions to take away. Concepts in brackets are less commonly encountered.

References
1. McAnena L, Knowles SJ, Curry A, Cassidy L. Prevalence of gonococcal conjunctivitis in adults and neonates. Eye (Lond) 2015;29(7):875-80.
2. Stenberg K, Mårdh P‐A. Chlamydial conjunctivitis in neonates and adults history, clinical findings and follow‐up. Acta Ophthalmol 1990;68(6):651-7.
3. Rackstraw S, Viswalingam ND, Goh BT. Can chlamydial conjunctivitis result from direct ejaculation into the eye? Int J STD AIDS 2006;17(9):639-41.
4. Belga S, Gratrix J, Smyczek P, et al. Gonococcal conjunctivitis in adults: Case report and retrospective review of cases in Alberta, Canada, 2000-2016. Sex Transm Dis 2019;46(1):47‑51.
5. Poli M, Cornut PL, Janin H, et al. Using systemic corticotherapy for adult gonococcal keratoconjunctivitis: Three case reports. J Fr Ophtalmol 2010;33(10):718-23.
6. Oliveira I, Mouzinho A, Marques JG. Gonococcal orbital cellulitis. BMJ Case Rep 2019;12(7):e227787.
7. Chang K, Cheng VYW, Kwong NS. Neonatal haemorrhagic conjunctivitis: A specific sign of chlamydial infection. Hong Kong Med J 2006;12(1):27‑32.
8. Kanski JJ: Clinical Ophthalmology. A Systematic Approach. 7th edition. Elsevier Health Sciences; 2011.
9. Nwokolo NC, Dragovic B, Patel S, et al. 2015 UK national guideline for the management of infection with Chlamydia Trachomatis. Int J STD AIDS 2016;27(4):251-67.
10.Fifer H, Saunders J, Soni S, et al. 2018 UK national guideline for the management of infection with Neisseria Gonorrhoeae. Int J STD AIDS 2020;31(1):4-15.
11.Martins TGdS, Peng G, Andrade E Nascimento, et al. Corneal complication caused by gonococcal conjunctivitis. Einstein (São Paulo) 2015;13(3):474. Available at:
www.scielo.br/scielo.php?script=sci
_arttext&pid=S1679-45082015000300474
COMMENTS ARE WELCOME





