
Thyroid eye disease (TED) is an autoimmune condition with a spectrum of signs and symptoms, usually associated with Graves’ hyperthyroidism. The diagnosis is based on history and physical examination but there are further investigations that can aid diagnosis if unclear. TED is the most common cause of unilateral and bilateral proptosis in adults and most frequently presents in women aged 30 to 50 years.
Despite this, the severity of TED is worse in men and in patients who are first diagnosed over 50 years old [1,2]. The estimated incidence of TED is 16 women and three men per 100,000 population per year [3,4]. There are multiple risk factors including cigarette smoking, older age at diagnosis of Graves’ hyperthyroidism, longer duration of Graves’ hyperthyroidism, uncontrolled thyroid dysfunction, and prior radioactive iodine treatment [3,5]. TED follows a biphasic course: a progressive / active phase which can last up to three years, followed by a stable or inactive phase [6,7]. The ophthalmic manifestations vary from mild to sight-threatening and the management varies from supportive to surgical depending on the severity. Clinical features include eyelid retraction, periorbital oedema, conjunctival injection and chemosis, proptosis, extraocular muscle restriction, exposure keratopathy and optic nerve compromise [8]. This variety in clinical features means there can be a range of differential diagnoses such as allergic conjunctivitis and orbital tumors, thus it is important to approach TED with a multidisciplinary approach.
The underlying molecular mechanism to the autoimmune process in TED is multifaceted. In Graves’ disease, there is over-expression of thyroid stimulating hormone receptor (TSHR) in the retrobulbar tissue. When TSHRs are activated, the orbital fibroblasts proliferate and secrete inflammatory cytokines and hydrophilic hyaluronan in the interstitial space. This causes large osmotic pressure gradients in the orbit leading to fluid accumulation. Additionally, some orbital fibroblasts differentiate into mature adipocytes, causing orbital adipose tissue expansion. This accumulation leads to atrophy, fibrosis and sclerosis of the extraocular muscles and can cause restrictive strabismus [1,2,9].

As aforementioned, TED can manifest in a spectrum of symptoms and signs. There are multiple classification tools that are used to classify TED. The first tool is a mnemonic – ‘NO SPECS’, which was created in 1969 and outlines the clinical signs of TED (Table 1), however, it is not a good indicator of severity and progression of TED [10]. In 1989, Mouritis and colleagues created the Clinical Activity Score (CAS) which characterises active and inactive stages, as seen in Table 2 [11]. Most recently, in 2008, the European Group on Graves’ Orbitopathy (EUGOGO) updated their management guidelines in view of clinical trials which are summarised in Table 3 [12].

Clinical assessment of TED involves clinical history, examination, biochemistry and imaging if indicated. Common clinical signs are upper eyelid retraction, conjunctival and caruncle injection and / or oedema, eyelid oedema and / or erythema with diurnal variation, ocular motility disruption or strabismus and proptosis [4]. Clinical evaluation for TED focuses on determining clinical activity and severity by assessing visual acuity, pupils, colour vision, extraocular movements, visual fields, exophthalmometry, external eyelid evaluation, slit-lamp examination and dilated fundus exam. Additional diagnostic testing can include a biochemistry screen for thyroid dysfunction, although it is important to note some patients with TED can be euthyroid [13]. If this is the case, further laboratory tests can be done such as TSH receptor antibodies (TRAb), thyroid stimulating immunoglobulins (TSI) and thyroid peroxidase antibody (TPO) [4].
Imaging studies of the orbit such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI) can help confirm TED whilst excluding other differential diagnoses. CT without contrast is most popular as it shows bony anatomy of the orbit and is more cost effective compared to MRI. CT orbits can show you characteristic TED changes such as enlargement of the extraocular muscle bellies and sparing of the tendons. Typically, there is asymmetrical bilateral involvement of the extraocular muscles affected in the following pattern: inferior recti, medial recti, superior recti, lateral recti and the obliques. If compressive optic neuropathy from orbital apex crowding is suspected, MRI is the gold standard imaging choice [14].
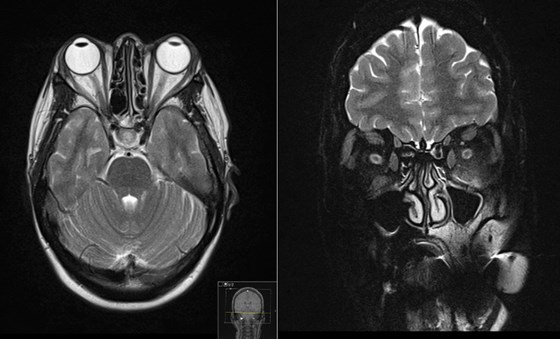
Figure 1: MRI orbit showing bilateral proptosis. There is associated thickening of the inferior, superior and medial extra ocular muscles. There is sparing of the lateral extraocular muscles bilaterally. There is minor high-signal associated with the left inferior rectus muscle. There is increase in the volume of the intra and extraconal fat bilaterally. Limited views of the intracranial contents are within normal limits.

Figure 2: MRI orbit showing mild bilateral proptosis and conal fat hypertrophy. There is there is small volume fluid around the left inferior rectus muscle and inflammatory change within the adjacent intra- and extraconal fat. There is also small volume fluid around the right inferior rectus muscle and minor inflammatory change to a lesser degree. All four ocular muscle bellies are symmetrically slightly enlarged but return normal signal. No discrete collections are seen.
Optimal management of thyroid dysfunction requires a multidisciplinary approach. Primary treatment goals are to restore and sustain a euthyroid state. Main treatment options are anti-thyroid drugs, thyroidectomy and radioactive iodine. There is debate regarding use of radioiodine as it can exacerbate TED, and some recommend the use of corticosteroids following radioiodine therapy to reduce risk of development / progression of TED [15,16]. Smoking cessation is the most important modifiable risk factor in prevention and progression of TED. EUGOGO recommends smoking cessation for all TED patients regardless of severity [17]. Conservative treatment for TED includes preservative free ocular lubrication, moisture chambers and taping eyelids for dry eye syndromes.
Sunglasses help with photosensitivity and glare [2]. If diplopia is present in the active phase, primes or monocular occlusion can help. If diplopia is present in the inactive phase, prism correction can be used in the patient’s glasses. Botulinum toxin injections to the levator palprebrae superioris and Muller muscle complex can also be trialed to reduce upper eyelid retraction [15,16]. Orbital radiation has been used for TED, however,r its role remains controversial. It is usually well tolerated and safe but is relatively contraindicated in patients under 35 years or with vascular disease. Orbital radiation reduces the proliferation rate of orbital fibroblasts thus reducing oedema [18]. Patients with compressive optic neuropathy and / or severe corneal exposure have sight-threatening TED. Recognition of these patients is important for timely management. Unexplained deterioration of vision, altered colour vision in one or both eyes, globe subluxation, corneal opacification, recent development of choroidal folds and optic disc oedema suggest sight-threatening thyroid eye disease.
The EUGOGO recommendation for patients with compressive optic neuropathy includes high-dose intravenous corticosteroids with urgent orbital decompression if there is little or no response to corticosteroids. In severe cases of corneal exposure, frequent topical lubricants may not be sufficient to prevent ulceration, thinning and perforation. In these cases, moisture chambers, topical cyclosporine, topical or subconjunctival corticosteroids, bandage soft contact lenses, therapeutic scleral contact lenses, amniotic membranes or tarsorrhaphy may be used to promote corneal healing [19,20]. Surgical rehabilitation is indicated for patients with moderate-to-severe inactive thyroid eye disease when there is a significant impact on visual function or quality of life.
The general surgical sequence employed in inactive thyroid eye disease is orbital decompression, followed by extraocular muscle surgery, with eyelid procedures performed last [13,16]. Exposure keratopathy can be treated with temporary tarsorrhaphy while awaiting orbital decompression. Extraocular muscle surgery (strabismus surgery) is considered in cases where diplopia persists or worsens following orbital decompression. Eyelid surgery is performed last for symptomatic eyelid retraction or asymmetric lid position with the goal of maintaining adequate corneal coverage [16].
References
1. Douglas RS, Gupta S. The pathophysiology of thyroid eye disease: implications for immunotherapy. Curr Opin Ophthalmol 2011;22:385-90.
2. Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of graves ophthalmopathy. Med Clin North Am 2012;96:311-28.
3. Perros P, Crombie AL, Matthews JN, et al. Age and gender influence the severity of thyroid-associated ophthalmopathy: a study of 101 patients attending a combined thyroid-eye clinic. Clin Endocrinol 1993;38:367-72.
4. Phelps PO, Williams K. Thyroid eye disease for the primary care physician. Dis Mon 2014;60:292-8.
5. Khong JJ, Finch S, De Silva C, et al. Risk factors for Graves' orbitopathy; the Australian Thyroid-associated Orbitopathy Research (ATOR) Study. J Clin Endocrinol Metabol 2016;101:2711-20.
6. Dolman PJ. Evaluating Graves' orbitopathy. Best Pract Res Clin Endocrinol Metabol 2012;26:229-48.
7. Barrio-Barrio J, Sabater AL, Bonet-Farriol E, et al. Graves' Ophthalmopathy: VISA versus EUGOGO Classification, Assessment and Management. J Ophthalmol 2015;2015:249125.
8. Menconi F, Marcocci C, Marino M. Diagnosis and classification of Graves' disease. Autoimmun Rev 2014;13:398-402.
9. Shan SJ, Douglas RS. The pathophysiology of thyroid eye disease. J Neuro-Ophthalmol 2014;34:177-85.
10. Werner SC. Classification of the eye changes of Graves' disease. Am J Ophthalmol 1969;68:646-8.
11. Mourits MP, Koornneef L, Wiersinga WM, et al. Clinical criteria for the assessment of disease activity in Graves' ophthalmopathy: a novel approach. Br J Ophthalmol 1989;73:639-44.
12. Bartalena L, Baldeschi L, Dickinson AJ, et al. Consensus statement of the European group on Graves' orbitopathy (EUGOGO) on management of Graves' orbitopathy. Thyroid 2008;18:333-46.
13. Scruggs RT, Black EH. Thyroid eye disease with significant levator involvement and ptosis: a case report. Ophthal Plast Reconstruct Surg 2015;31:e153-e154.
14. van der Molen AJ, Thomsen HS, Morcos SK. Effect of iodinated contrast media on thyroid function in adults. Eur Radiol 2004;14:902-7.
15. Thyroid eye disease: therapy in the active phase. J Neuroophthalmol 2014;34:186-97.
16. Verity DH, Rose GE. Acute thyroid eye disease (TED): principles of medical and surgical management. Eye (Lond) 2013;27:308-19.
17. Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid 2010;20:777-83.
18. Kahaly GJ, Roesler HP, Kutzner J, et al. Radiotherapy for thyroid-associated orbitopathy. Exp Clin Endocrinol Diabetes 1999;107(Suppl 5):S201-S207.
19. Bartalena L, Baldeschi L, Boboridis K, et al. The 2016 European Thyroid Association/European Group on Graves' Orbitopathy Guidelines for the Management of Graves' Orbitopathy. Eur Thyroid J 2016;5:9-26.
20. Marcocci C, Marino M. Treatment of mild, moderate-to-severe and very severe Graves' orbitopathy. Best Pract Res Clin Endocrinol Metabol 2012;26:325-37.
Declaration of competing interests: None declared.
COMMENTS ARE WELCOME






