Contemporary laser in situ keratomileusis (LASIK) is safe and effective. It remains the dominant intervention in routine refractive surgery for a good reason: predictable results, rapid visual recovery, and relatively simple strategies for revision treatment. Over 95% of patients are satisfied with their result [1], and success is now defined in a large part by incremental gains in satisfaction through anticipating problems and treating them effectively.
What you say in surgery is as important as what you do. Patients need to understand risks, benefits and side-effects for a procedure from the get-go.
The best way to explain risk in LASIK is in terms of the two ends of the spectrum. What is the worst likely scenario (lamellar keratoplasty)? What is the most common thing that you might run into other than a completely smooth recovery (minor revision)? Lamellar keratoplasty is rarely required, whereas minor revisions are common. For the patient, it is reassuring to know that there is almost always a viable pathway back to good vision after complications in LASIK. It is also reassuring to be clear that minor revisions are very much part of the LASIK package, and are often required in order to get the best results.
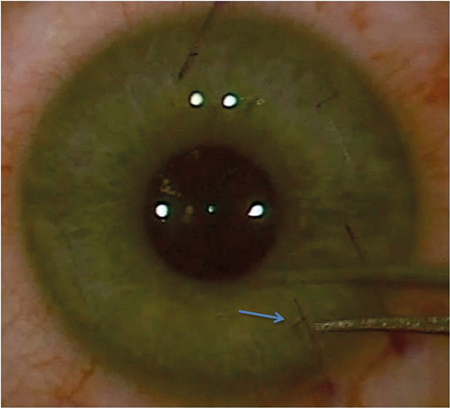
Figure 1: Gentian violet runs into the flap side cut (arrow) after irrigation with balanced
salt solution highlighting the point for a clean forceps entry with minimal epithelial trauma.
If problems are encountered, patients need a clear treatment plan outlined to them. If a problem may not be fixed by the first intervention, patients need to know this at the start. Then they are on-board with you when further work is needed. Walk patients through a stepladder approach to the management of complications with the least invasive intervention on the bottom rung, moving on all the way to through to lamellar keratoplasty. Let them know there is a safety net at every stage.
Minor revision
Minor revision falls into two broad categories:
1) interventions to promote good healing
2) enhancement laser treatments.
All these interventions feel much the same to the patient. They all involve additional surgery under topical anaesthetic with a similar recovery pattern to the original LASIK treatment.
With good nomogram development and modern laser systems, routine LASIK enhancement treatment rates are as low as 2-3%. But flap revisions are common, and up to 10% of patients will require some form of minor revision after surgery.
Day one review is pivotal. Flap dislocations are common after LASIK, and almost always occur in the first few hours after surgery. Telltale signs are microstriae, an edge gap with epithelial infilling, and meibomian debris in the interface.
Dislocations are easily corrected at the slit-lamp. The eye is prepared with topical anaesthetic and povidone iodine 5%. Put some paper towels under the patient’s chin, and use a disposable Barraquer speculum to maintain the palpebral aperture. Place this under the top lid first, and ask the patient to look down then up to catch the lower lid easily. Ask the patient to fix a target object with the other eye – your ear or something behind you to the left or right in the consulting room, depending on the eye position that you need for the best access. The flap can then be reflected using disposable curved tying forceps to expose the interface and debride the epithelium up to the side cut. Most flap dislocations are partial, and only require a partial lift on the side of the flap edge retraction. After epithelial clearance, the flap is replaced and sub-flap irrigation is used to float the flap back into position and clear any meibomian debris using a ‘squeezy-bottle’ of balanced salt solution (BSS) with a short cannula. In a partial lift, it is important to break interfacial adherence over the entire area beneath any microstriae.
The flap can then be ironed back into place easily leaving no edge gap, as in the primary procedure using wet, then semi-hydrated, arrow tip sponges. In a complete flap dislocation, work down the flap with two counteracting arrow tip sponges ironing away from the centre to the periphery. Semi-hydrated sponges should be dry enough to provide the traction required to iron out any edge gap, but wet enough to minimise epithelial trauma. To complete the revision, instill unpreserved broad-spectrum topical antibiotics and steroids and a bandage contact lens. Review the patient after an hour or later, then again the next day. Immediately after revision, broad furrows in the epithelium, or ‘ghost striae’ are visible where stromal striae have been opened in flap repositioning. These disappear with epithelial remodeling by day one.
Slit-lamp flap revisions avoid any hold-ups over space in the operating theatre, and give a better view of the interface than the diffuse light of an operating microscope. This is particularly useful for clearance of localised inflammation or interface debris.
Look for a clean interface, no striae and no edge gap the day after minor revisions, and let the patient know that repeat intervention is common. Many later problems can be avoided by an aggressive approach to obtaining a perfect flap position, with no striae and no interface debris.
Figure 2a: Annular pattern of diffuse lamellar keratitis on day one after surgery.

Figure 2b: Central toxic keratopathy (CTK) presenting on day six after surgery.

Figure 2c: The same case after resolution of the CTK at one year after surgery.
Epithelial ingrowth
In a review of 1000 consecutive cases [2], we identified a clear statistical association between epithelial trauma at surgery (or after), interface inflammation, and subsequent epithelial ingrowth secondary to inflammatory chemotaxis in from the interface. Gearing your primary surgical technique to avoiding epithelial trauma is the best way to avoid this sequence. A good tip is to mark the flap edge at the intended site of your forceps entry with gentian violet then irrigate with BSS before the flap lift. The BSS causes the gentian violet to run into the side cut promoting visualisation for a clean forceps entry.
Keeping the flap surface well hydrated prior to the spatula pass after breaking into the side cut also helps to maintain epithelial integrity during the flap lift. For enhancement treatments, break into the original side cut at the slit-lamp using sharp dissection with a 25 gauge needle under topical anaesthetic. It is then relatively easy to open into the flap interface under the laser microscope with suture tying forceps, then lift the flap with a rhexis technique rather than spatula sweeps to minimise epithelial disturbance.
Bacterial antigen deposition is another common cause of interface inflammation, often accompanied by multifocal inflammatory infiltrates at the flap edge. Pre-treat any meibomian dysfunction prior to surgery, and cover the perioperative period with systemic doxycycline 100mg once daily, starting two weeks before surgery and continuing for one month after.
Epithelial ingrowth is normally present by one week after surgery, and is unlikely to occur in the absence of interface inflammation or a flap dislocation visible on day one, or epithelial trauma at surgery. Patients with any abnormal findings on day one will need to be reviewed one week after surgery with the aim of intercepting ingrowth at an early stage.
Flap revision at the slit-lamp to debride ingrowth is exactly as for flap dislocation on day one. The flap is partially reflected in the zone of ingrowth up to the point at which a clean interface at the side cut is identified on both sides. The epithelium is then brushed away easily from the interface or the underside of the flap. At one week, there is not yet any fibroblastic transformation in the ingrowing cells, and removal is relatively straightforward. The inflammatory drive to interfacial ingrowth has normally resolved substantially at that point, after a week of intensive topical steroid treatment. Again, however, the main message to the patient at that stage is that repeat intervention is often required for the best results. See the patient again one week later and repeat the flap lift if necessary, and continue this cycle until the interface is clean.
Identifying early stage, pre-fibrotic ingrowth is a bit like hearing mitral stenotic murmurs – there is an art to it. But once you tune into the signs, you will quickly get a feel for the value of early intervention. A subtle, ground glass interface opacification with an irregular leading border growing in from the flap edge is usually visible at the site of a previous lift, localised inflammation or epithelial trauma where ingrowth is present.
Interface opacity after day one
Diffuse lamellar keratitis (DLK) is closely analogous to TASS syndrome in cataract surgery, and is always present on day one. Later stage focal inflammation is infection until proven otherwise. Infection occurs in approximately 1/3000 cases [3], with gram positive cocci predominant. The 2005 ASCRS antimicrobial guidelines should be followed in the management of these cases with contemporary investigative adjuncts including confocal microscopy and polymerase chain reaction (PCR) to help identify fungal infection at an early stage.
An annular pattern of DLK seen on Day 1 can be a prelude to central toxic keratopathy. This syndrome presents with focal opacification and thinning of the stromal bed with ‘mud crack’ interfacial striae, typically on day four to seven after surgery. The aetiology of CTK remains unclear, but it occurs in the context of interface inflammation, and anything other than mild, diffuse DLK should be washed out at the interface with BSS on day one. Treatment of CTK is conservative, with no flap lift, and cessation of topical steroids after resolution of the associated interface inflammation [4]. Confocal microscopy shows keratocyte apoptosis back to Descemets membrane at the site of the opacity in CTK, with repopulation and at least partial resolution of the initial irregular hyperopic shift between three and 12 months after onset. There are no reports of recurrent CTK, and enhancement treatment once the refraction has stabilised can be performed if necessary.
Interface fluid syndrome is a trap for the unwary, which is now well recognised, and should always be considered in cases with ‘persisting interface inflammation’ and paradoxical low pressure six to 12 weeks post surgery. Ocular coherence tomography (OCT) sections are diagnostic, and the interfacial fluid can normally be visualised at the slit-lamp. The fluid collection is driven by raised intraocular pressure, typically in steroid responsive patients. Substitution of topical steroids with anti-glaucomatous medication normally effects a rapid resolution.
Figure 3a: Intractable fibrotic epithelial ingrowth with irregular astigmatism and peripheral stromal melting.
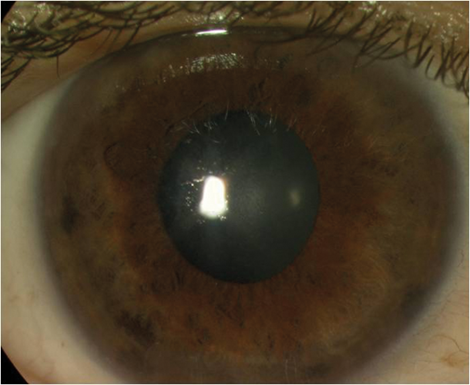
Figure 3b: The same case two weeks after flap amputation (uncorrected distance visual acuity 6/6).
Late flap trauma and intractable ingrowth
Late flap dislocation is uncommon, and requires a significant sheer injury. Although the potential for late flap dislocation is often cited as a reason for not doing LASIK, flap amputation is safe, effective and restores good vision quickly after a partial flap avulsion. Flap amputation can also be a useful intervention in cases of intractable post-fibrotic epithelial ingrowth, which are typically associated with an extensive peripheral flap melt. An aggressive approach to ingrowth at the pre-fibrotic stage normally avoids recalcitrant problems later. But flap amputation is an effective remedy if YAG laser ablation has been unsuccessful and visually significant or progressive problems with ingrowth remain.
Flap amputation sounds extreme, but the reality in the post-femtoLASIK era is that you are only removing just over 50 microns of stromal tissue. The residual stromal bed remains the principal substrate for biomechanical integrity, and there have been no reports of any long-term problems where flap amputation has been required.
Intercepting ectasia
Contemporary corneal tomography techniques help to identify ectasia at an early stage. Always look first at the Belin Ambrosio posterior corneal maps in cases with myopic or astigmatic results after LASIK. In contrast to the anterior maps, which are always altered after treatment, the posterior maps in the right hand column should be normal. Ectasia is progressive by definition, but where doubt exists, comparison maps for the posterior corneal profile in particular are helpful in monitoring for change.
Ectasia is less common now that risk factors are better understood [5] and patients are more effectively screened prior to surgery, and early stage recognition opens the door to effective treatment with combined wavefront guided transepithelial photorefractive keratectomy (PRK) and corneal collagen cross-linking (CXL). Confining the treatment to the flap stroma will have little impact on biomechanical integrity, and removal of flap stroma may even help to sink the zone of cross-linked tissue into the residual stromal bed. As strategies for thin bed cross-linking evolve, combined TransPRK and CXL treatments will gain traction in the treatment of ectasia. At minimum, conventional epithelium off CXL can be applied to help arrest disease progression, and intracorneal ring implantation can be effective for gross shape correction prior to CXL in cases with significant coma. But where ectasia is rapidly progressive, lamellar keratoplasty may be required.
In ectasia, prevention is undoubtedly better than cure, and the basic rule is ‘don’t tread the margins’ in terms of the percentage thickness treated or the corneal tomographic indices in either LASIK or SMILE. ICLs are a great alternative where increased risk exists [5], and surface laser treatments combined with light touch CXL are becoming better understood.
Figure 4: Rapid onset corneal ectasia after surface laser enhancement treatment four months after primary LASIK.
This patient required a deep anterior lamellar keratoplasty followed by ICL implantation
to restore the uncorrected distance vision to 6/7.5.
Continuity of care Good
communication, good patient selection, anticipation of problems and aggressive early treatment are the cornerstones of high quality care in LASIK. Attention to detail in each of these areas, together with good surgical training, good equipment and the right environment, will help to reduce the relatively small numbers of dissatisfied patients in LASIK still further. The Royal College of Ophthalmologists has recently published revised Professional Standards in Refractive Surgery aiming to drive additional gains in quality of care. LASIK is already an extremely safe procedure. A clear understanding what to do when problems occur can make it safer still.
References
1. Solomon KD, Fernandez de Castro LE, Sandoval HP, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology 2009;116(4):691-701.
2. Watson SL, Bunce C, Allan BD. Improved safety in contemporary LASIK. Ophthalmology 2005;112(8):1375-80.
3. Donnenfeld ED, Kim T, Holland EJ, et al. ASCRS White Paper: Management of infectious keratitis following laser in situ keratomileusis. J Cataract Refract Surg 2005;31(10):2008-11.
4. Sonmez B, Maloney RK. Central toxic keratopathy: description of a syndrome in laser refractive surgery. Am J Ophthalmol 2007;143(3):420-7.
5. Ambrosio R Jr, Ramos I, Lopes B, et al. Ectasia susceptibility before laser vision correction. J Cataract Refract Surg 2015;41(6):1335-6.
Declaration of competing interests: The author has received financial support to attend expert user meetings organised by Schwind Eye Tech Solutions GmbH (manufacturer of excimer lasers) and Staar Surgical Inc (ICL manufacturer).
COMMENTS ARE WELCOME